JMP Pharma Pvt Ltd
How JMP Pharma engages in collaboration with clients.
Our objective
Phase I: Establishing a robust and safe process. Phase II: Identifying target synthetic methodologies, selecting synthesis routes, and determining discrete parameters following Phase II trials. Phase III: Optimizing process parameters, utilizing Design of Experiments (DoE) as necessary.
Synthesis and process development
Phase I: Assessing route viability, controlling discrete parameters, and managing impurities (organic and mutagenic) for API compliance with specifications, alongside necessary physical characteristics. Phase II: Continuation of impurity studies, focusing on physical characteristics, crystallization, and milling investigations. Phase III: Determining critical parameters, establishing Normal Operating Ranges (NOR) and Process Alarm Ranges (PAR).
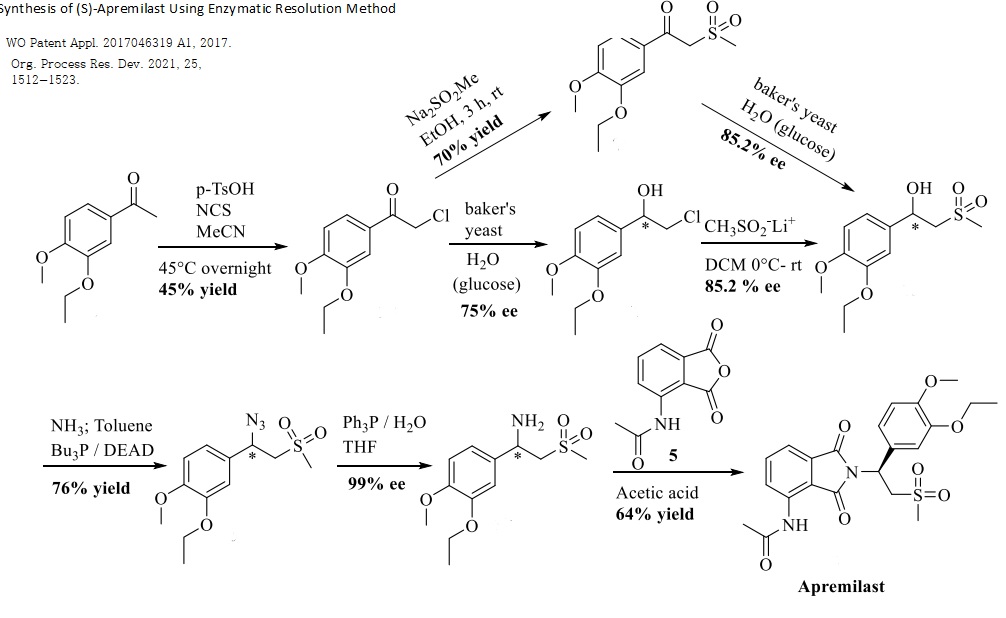
Services
Our team of exceptionally skilled scientists holds Ph.Ds from renowned Indian Institutes or postdoctoral experience from overseas, and Masters in chemistry complemented by years of hands-on expertise in process R&D of pharmaceutical intermediates, NCEs (Medicinal Chemistry) development, pharmaceutical impurities, and custom synthesis of specialty chemicals.

“What is the point of being alive if you don’t at least try to do something remarkable?”
JANET MORRIS
Other projects
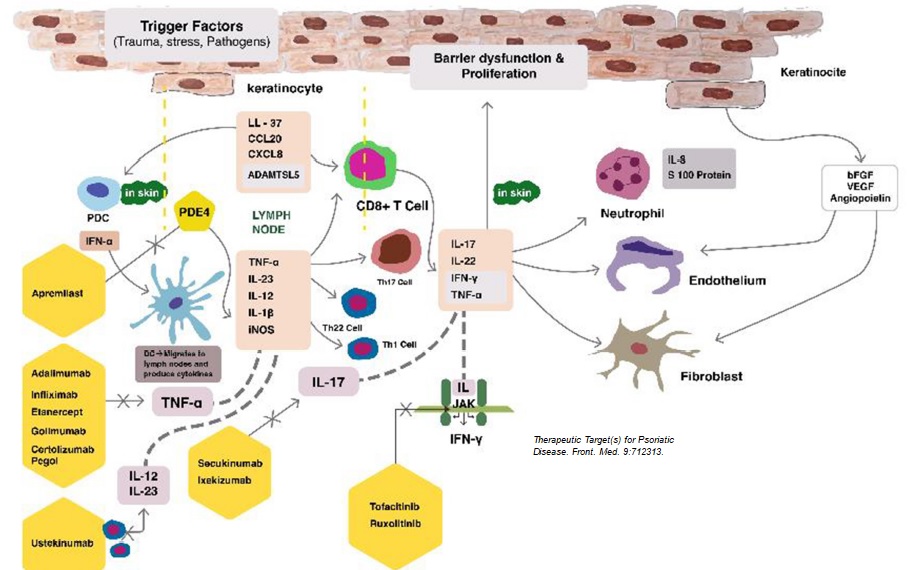
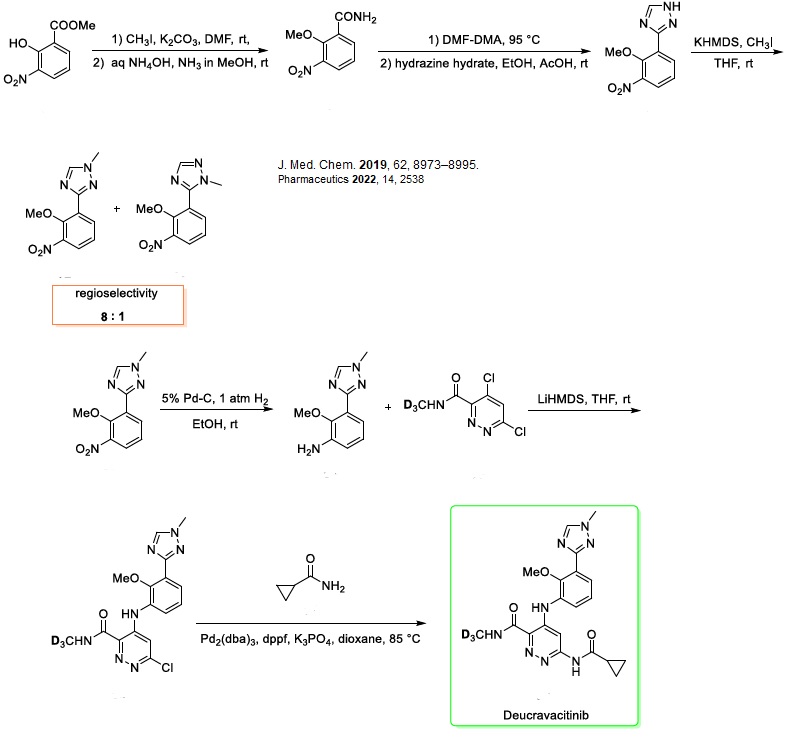
Let’s work together on your
next web project
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus
nec ullamcorper mattis, pulvinar dapibus leo.